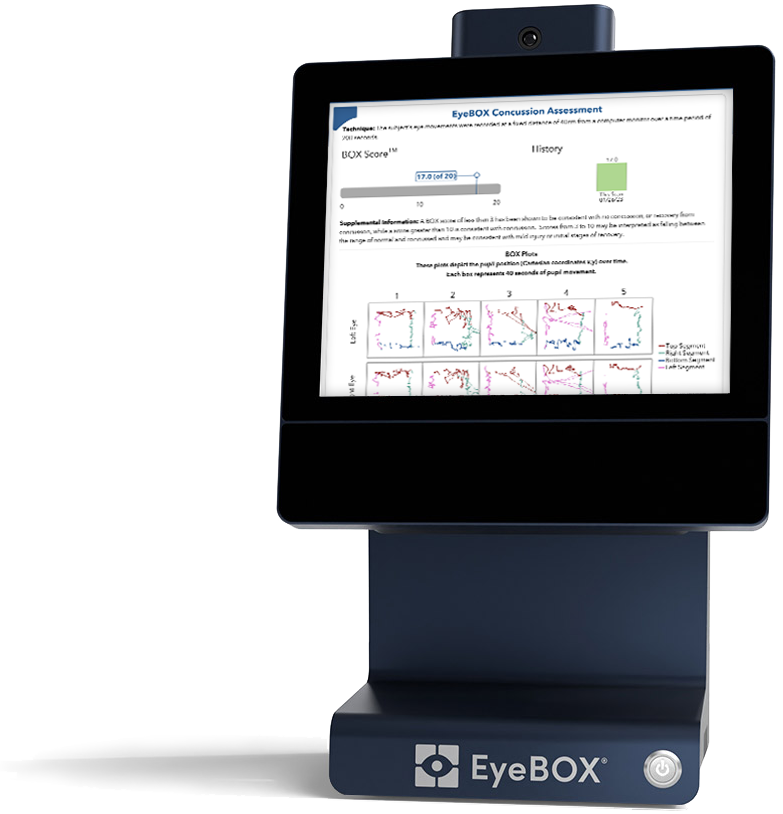
Provide your patients with immediate concussion screening results.
Gain Objective Insights
EyeBOX® is FDA-cleared to aid specifically in the diagnosis of concussion by measuring abnormalities in eye-movements. It’s baseline-free, non-invasive, and done in less than 4 minutes.
Communicate Clearly
The EyeBOX® report is available ten seconds after the test is complete and helps you communicate clearly with patients, their family, payers, and other providers.
Increase Diagnostic Consistency
Use clinically-validated EyeBOX® technology to assess concussion more consistently across patients and across providers in your organization. EyeBOX® has been the subject of 14 peer-reviewed studies.
Provide your patients with immediate concussion screening results.



Gain Objective Insights
EyeBOX® is FDA-cleared to aid specifically in the diagnosis of concussion by measuring abnormalities in eye-movements. It’s baseline-free, non-invasive, and done in less than 4 minutes.
Communicate Clearly
The EyeBOX® report is available ten seconds after the test is complete and helps you communicate clearly with patients, their family, payers, and other providers.
Increase Diagnostic Consistency
Use clinically-validated EyeBOX® technology to assess concussion more consistently across patients and across providers in your organization. EyeBOX® has been the subject of 14 peer-reviewed studies.

Gain Objective Insights
EyeBOX® is FDA-cleared to aid specifically in the diagnosis of concussion by measuring abnormalities in eye-movements. It’s baseline-free, non-invasive, and done in less than 4 minutes.

Communicate Clearly
The EyeBOX® report is available ten seconds after the test is complete and helps you communicate clearly with patients, their family, payers, and other providers.

Increase Diagnostic Consistency
Use clinically-validated EyeBOX® technology to assess concussion more consistently across patients and across providers in your organization. EyeBOX® has been the subject of 14 peer-reviewed studies.
Elevate your services and be at the forefront of concussion diagnosis.



A visionary breakthrough in concussion diagnosis.
Hear what our customers are saying about EyeBOX®

Hear how EyeBOX helps Kyle with treatment of concussions
Hear why Lisa and her team acquired EyeBOX

Wide Patient Range
Ability to assess patients from ages 5 to 67.
Objective Results
EyeBOX® delivers an objective score and eye-tracking metrics immediately following the test.
Easy for the Patient
Using EyeBOX® is as simple as asking your patient to watch a short, engaging video.
Non-Invasive
Non-invasive process that doesn’t require lab work for confirmation.
Reimbursable
The EyeBOX® test is reimbursable.
Learn how EyeBOX® works

"Oculogica is exactly the type of dynamic, leading-edge company we envisioned being part of Titletown Tech... Oculogica's innovation benefits not only the sports community, but health care overall."
Mark Murphy
Green Bay Packers President and CEO
"This is an important achievement for the field, which has had to work with the lack of objective measures until now. The EyeBOX as part of an objective, multimodal assessment will eventually be the new state of the art."
Christina Master, MD, FAAP, CAQSM, FACSM
Professor of Clinical Pediatrics, University of Pennsylvania; Attending Physician, Children's Hospital of Philadelphia
By minimizing the guesswork involved in diagnosing concussions, Kuenzi says, that kind of tool can be a big help to coaches trying to protect players and defend a sport increasingly seen as too dangerous. “You’re talking the brain,” he says. “We’re not gonna mess around with that.”
Steve Kuenzi
Beaver Dam High School Head Football Coach
“I’m so grateful. I didn’t know what to do. The trainer wouldn’t see him because we didn’t have a baseline test. I felt really stuck not knowing exactly what was going on. It was so reassuring. It was really simple, and eased his anxiety.”
Janel
Mother of high school football player
“This is the most current technology available to more precisely assess ocular changes after a concussion, and this data can help us understand which patients would benefit from earlier treatment. I appreciate any tool which helps me eliminate subjectivity in a diagnosis.”
Elizabeth Pieroth, PsyD, ABPP, MPH
Director of the Concussion Program, Midwest Orthopaedics at Rush Assistant Professor, Rush Medical College
“I was pleasantly surprised at how quickly I was able to receive a diagnosis after taking the EyeBOX test and with the proper diagnosis, I was assured to receive the right care.”
Cassidy
EyeBOX patient in ColoradoSchedule a Virtual Demonstration

Fill out the form below to request a virtual demonstration
In order to receive information about your Personal Data, the purposes and the parties the Data is shared with, contact the Owner. For more information and to understand your rights, you can also view the complete version of this privacy policy, by clicking the link provided above